Information from the Department of Health and Social Care regarding the urgent Clexane 40mg injection supply issue:
Sanofi contacted NHS England and DHSC yesterday to inform us that there has been a quality issue with their most recent batch of Clexane 40mg injection that was due to arrive this week in the UK. It is anticipated that all wholesalers will be out of stock of Clexane 40mg injection by the end of the week with an anticipated resupply date of week commencing 18 February 2019. This date may be brought forward to end of January/early February if the current batch passes further QA testing.
Alternative options:
Clexane (Sanofi) Imported stock – available from 23rd January 2019
-
Over the past 2 weeks Sanofi have imported Clexane 40mg stock from Italy to help cover an anticipated shortfall in stock. This product is imported under a batch specific variation to the UK and is therefore classed as licensed in the UK.
-
Sanofi are working with the MHRA and this product should be available to order from the 23rd January 2019. Once available it can be ordered via the usual routes.
-
Key differences are as follows:
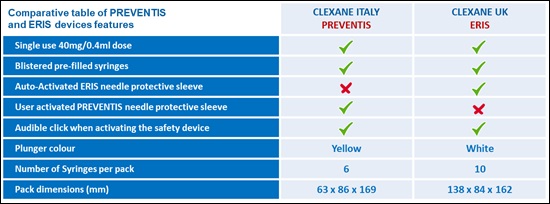
-
The most important difference between the Italian and UK preparations is the difference in the needle guard device. To deploy the Preventis needle shield on the Italian syringes, users will need to firmly push the plunger after completing the injection. The user will hear an audible “click” to confirm the activation of the protective sleeve and the protective sleeve will automatically cover the needle. Patients and HCPs will need to be trained on this new device; instructions for use can also be found within the PIL. This product will NOT be over-labelled in English but an English PIL will be included in the pack. Artwork is attached which gives further detail of what the packaging and device looks like.
Alternative products
We have been in contact with the suppliers of Enoxaparin biosimilar agents and the following information is available:
Inhixa 4,000 IU (40mg) in 0.4ml solution for injection pre-filled syringe (supplied by Techdow)
Arovi 4,000 IU (40mg) in 0.4ml pre-filled syringe (supplied by Rovi Biotech)
-
Rovi Biotech supply Arovi (enoxaparin) and have confirmed that they have sufficient Rovi 40mg injection to cover additional demand in primary care.
-
If you wish to switch Clexane 40mg to Arovi please see attached supporting / educational material and link to further online material: https://www.rovi.es/en/biosimilar-de-enoxaparina
-
For any queries or to order training material please contact: Blanca Esteban, [email protected] 0203 642 06 77.
-
Orders for Arovi can be place via Alliance Healthcare.
Action Required
-
Review your current Clexane 40mg injection patients and determine if they have sufficient supplies until the next Clexane 40mg stock becomes available – 23rd January for the Italian imported (UK licensed) stock and currently 18th February for further UK Clexane 40mg stock.
-
We would suggest GPs and pharmacies work closely to discuss local management options.
-
If product is required before 23 January 2019 – use one of the suggested alternatives above.
Alternative Clexane presentations remain available however, Sanofi cannot support increased ordering of any other presentations to support the shortfall in 40mg syringes. Pharmacies are asked to continue to order all other strengths in line with historical demands, and reminded that NHS Purchases will be monitored during this period.
Please be assured that we are working closely with NHS England, Sanofi, the MHRA and all other suppliers to ensure that the situation resolves as quickly as possible and that patients can continue to obtain supplies of enoxaparin 40mg injection.
Further information: